Overall
|
|
Countermeasure
 |
Biological and chemical threat agents, such as emerging viruses, biological toxins, and chemical nerve agents, pose significant risks to public health. Despite their diverse molecular structures and distinct mechanisms of action, these agents share a common feature: their interaction with host cell membranes. This functional similarity provides a foundation for designing broad-spectrum preventative and therapeutic solutions. Cellular nanoparticles, which replicate all natural cellular receptors on their surface, offer a novel approach to counteracting these deadly agents. Acting as universal nanodecoys, they capture and neutralize target agents. For instance, using human lung epithelial cell membranes and macrophages, we developed cellular nanoparticles capable of binding to various virus species and families, including coronaviruses. These nanoparticles block viral entry into cells and inhibit infectivity. Additionally, cellular nanoparticles can neutralize a wide range of cytokines and autoantibodies, making them valuable for treating numerous immunological and inflammatory diseases. In this research area, we are actively developing broad-spectrum, ready-to-use therapies for deadly viruses and other threat agents, as well as innovative treatments for immunological and inflammatory disorders. |
Drug Delivery
 |
Modifying the surface of drug nanocarriers to enhance their compatibility with the body and improve their ability to target specific cells and tissues is a critical but unresolved challenge. Drawing inspiration from nature, we developed cellular nanoparticles by cloaking synthetic drug nanocarriers with membranes extracted directly from natural cells. This cell membrane coating provides the nanoparticles with complex immunomodulatory proteins, enabling them to evade the immune system for extended periods. As a result, these nanoparticles can circulate undetected in the bloodstream, efficiently delivering their drug payload. Additionally, by leveraging the natural interactions between cells, tissues, and organs, cellular nanoparticles can recognize and bind to specific target sites, achieving precise drug delivery. We further enhanced this capability by engineering cells to express specific receptors, allowing the production of genetically modified cellular nanoparticles for targeted drug delivery. In this research area, we are actively developing innovative cellular nanoparticle formulations for the targeted delivery of various payloads, including small molecules, proteins, and nucleic acids. We are also exploring the integration of these nanoparticles with microrobot systems to enable active drug delivery. |
Vaccine
 |
Vaccines are routinely used to enhance the body’s innate defenses by stimulating immune responses against disease-causing cells or organisms. Traditional vaccine formulations typically use weakened microbes, surface proteins, or virulence factors to train the immune system to recognize and neutralize similar biological agents upon future exposure. Recently, nanoparticles have gained attention for their unique ability to enhance vaccine potency, offering a safer and more effective approach to vaccine development. Our research focuses on developing nanovaccines and studying their interactions with the immune system. For instance, we created a novel cancer vaccine using cancer cell membrane-coated nanoparticles. By loading adjuvants into nanoparticles and coating them with autologous tumor cell-derived plasma membranes, we developed a personalized cancer vaccine that trains the immune system to target multiple tumor antigens without requiring prior antigen identification. In this research area, we are now expanding this cellular nanoparticle technology to pathogen membranes, aiming to develop robust outer membrane vesicle-coated nanoparticle vaccines for the prevention of a wide range of infectious diseases. |
Diagnostics
 |
The development of rapid and reliable analytical techniques for detecting key disease biomarkers in complex biological samples is crucial for advancing disease diagnostics. Conventional sandwich immunosensors rely on antibody recognition layers to selectively capture and detect target antigens. We introduced a novel bioelectronic affinity sensing strategy that employs natural cell membranes as recognition layers, offering a compelling alternative to antibody-based receptors in traditional immunoassays. The natural cell membrane coating preserves membrane proteins and lipids, endowing the coated devices with unique properties for interfacing with biological systems, such as capturing and isolating biological targets and preventing non-specific adsorption. In this research area, we focus on integrating natural cell membranes with established sensing platforms, such as field-effect transistors and immunosensors, to develop innovative biosensor systems for sensitive and accurate disease detection. |

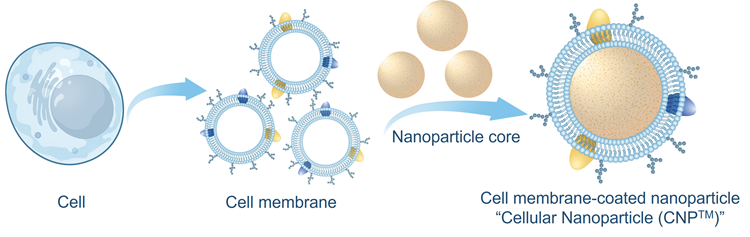 In 2011, our laboratory introduced a groundbreaking technology: cell membrane coating nanotechnology.
By using human red blood cell membranes to coat synthetic nanoparticles, we developed RBC-NPs for long-circulating drug delivery (2011 PNAS). Subsequently, we discovered that these RBC-NPs
could neutralize various bacterial toxins (2013 Nature Nanotechnology), autoimmune antibodies (2014 PNAS), and chemical nerve agents (2015 ACS Nano). Moreover, the captured toxins
could serve as potent antigens, enabling the development of innovative toxoid vaccines (2013 Nature Nanotechnology). This versatile platform technology has been successfully extended to
many other cell types. For example, we applied it to cancer cell membranes (2014 Nano Letters), platelet membranes (2015 Nature), and white blood cell membranes, including macrophages (2017 PNAS),
neutrophils (2018 Nature Nanotechnology), and T cells (2018 Advanced Materials). Furthermore, we demonstrated its application to pathogen membranes, creating bacterial membrane-coated nanoparticles
for antimicrobial vaccines (2015 Nano Letters). Additionally, we engineered cells to express specific receptors or remove unwanted ones, enabling the production of genetically modified
cell membrane-coated nanoparticles (2021 Science Advances, 2024 Nature Nanotechnology). To facilitate broader applications and clinical translations, these cell membrane-coated nanoparticles have been rebranded as
"Cellular Nanoparticles (CNPs)", a trademarked technology (CNPTM). As a new therapeutic modality, CNPs have advanced to clinical trials, with two Investigational New Drug (IND)
applications approved for human studies. While we are thrilled to see this technology moving from basic discovery to clinical application, our research remains focused on exploring new frontiers.
We aim to further develop and investigate innovative CNP-based solutions for countermeasure, drug delivery, vaccines, and diagnostics to address critical unmet medical needs.
In 2011, our laboratory introduced a groundbreaking technology: cell membrane coating nanotechnology.
By using human red blood cell membranes to coat synthetic nanoparticles, we developed RBC-NPs for long-circulating drug delivery (2011 PNAS). Subsequently, we discovered that these RBC-NPs
could neutralize various bacterial toxins (2013 Nature Nanotechnology), autoimmune antibodies (2014 PNAS), and chemical nerve agents (2015 ACS Nano). Moreover, the captured toxins
could serve as potent antigens, enabling the development of innovative toxoid vaccines (2013 Nature Nanotechnology). This versatile platform technology has been successfully extended to
many other cell types. For example, we applied it to cancer cell membranes (2014 Nano Letters), platelet membranes (2015 Nature), and white blood cell membranes, including macrophages (2017 PNAS),
neutrophils (2018 Nature Nanotechnology), and T cells (2018 Advanced Materials). Furthermore, we demonstrated its application to pathogen membranes, creating bacterial membrane-coated nanoparticles
for antimicrobial vaccines (2015 Nano Letters). Additionally, we engineered cells to express specific receptors or remove unwanted ones, enabling the production of genetically modified
cell membrane-coated nanoparticles (2021 Science Advances, 2024 Nature Nanotechnology). To facilitate broader applications and clinical translations, these cell membrane-coated nanoparticles have been rebranded as
"Cellular Nanoparticles (CNPs)", a trademarked technology (CNPTM). As a new therapeutic modality, CNPs have advanced to clinical trials, with two Investigational New Drug (IND)
applications approved for human studies. While we are thrilled to see this technology moving from basic discovery to clinical application, our research remains focused on exploring new frontiers.
We aim to further develop and investigate innovative CNP-based solutions for countermeasure, drug delivery, vaccines, and diagnostics to address critical unmet medical needs.